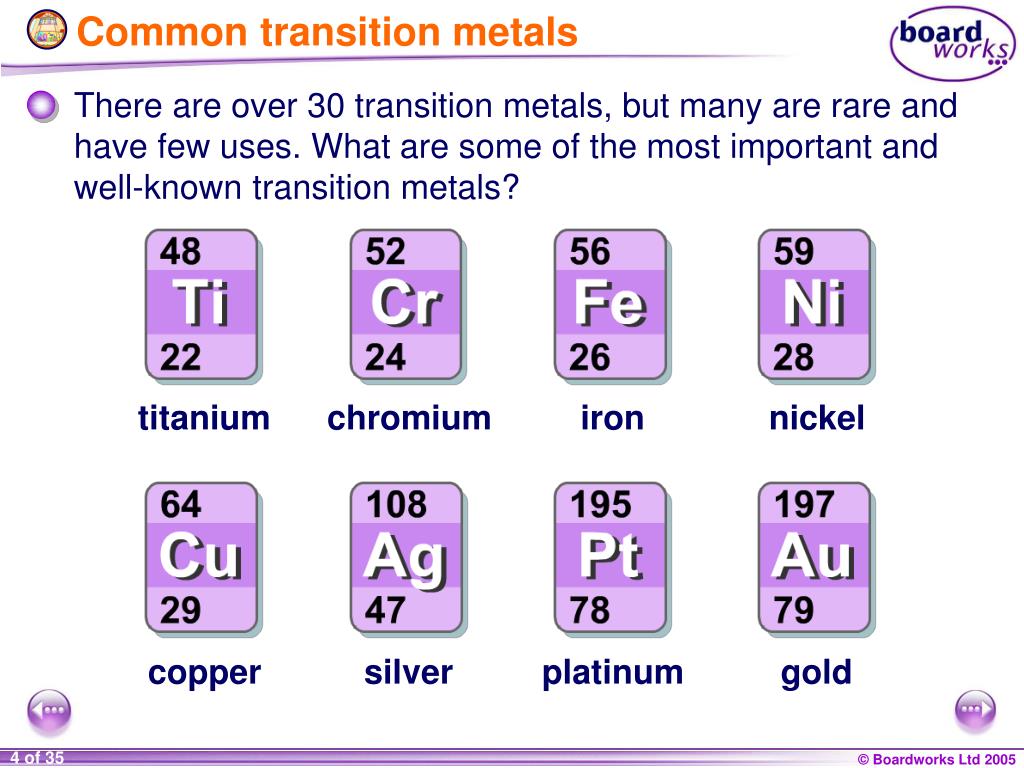
Transition Metals Short Form . transition metals are defined as those elements that have (or readily form) partially filled d orbitals. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal.
from www.slideserve.com
this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal.
PPT KS4 Chemistry PowerPoint Presentation, free download ID6307470
Transition Metals Short Form typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. typical properties of transition metals the existence of more than one oxidation state for each element in its. transition metals are defined as those elements that have (or readily form) partially filled d orbitals.
From www.breakingatom.com
The Transition Metals Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. transition metals are defined as those elements. Transition Metals Short Form.
From www.vedantu.com
Uses of Transition Metals Learn Important Terms and Concepts Transition Metals Short Form A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure,. Transition Metals Short Form.
From www.youtube.com
The Transition Metals YouTube Transition Metals Short Form typical properties of transition metals the existence of more than one oxidation state for each element in its. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. this page explains what a transition metal is in terms of its electronic structure,. Transition Metals Short Form.
From periodictableguide.com
Where are Transition Metals located on the Periodic Table? Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. A metal that is located in between group 2 and group 3 (labelled as group. Transition Metals Short Form.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6307470 Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. typical properties of transition metals the existence of more than one oxidation state for. Transition Metals Short Form.
From commons.wikimedia.org
FileTransition metals in the periodic table.png Wikimedia Commons Transition Metals Short Form typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. transition metals are defined as those elements that have (or readily form). Transition Metals Short Form.
From www.haikudeck.com
Transition Metals by Sarena Kinkel Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. this page explains what a transition metal is in terms of its electronic structure, and then. Transition Metals Short Form.
From www.slideserve.com
PPT Lecture 4 Transition MetAL Organometallics LIGANDS PowerPoint Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. typical properties of transition metals the existence of more than one oxidation state for each element in its. A metal that is located in between group 2 and group 3 (labelled. Transition Metals Short Form.
From www.yumpu.com
Transition Metals form Po Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. typical properties of transition metals the existence of more than one oxidation state for. Transition Metals Short Form.
From chem.libretexts.org
Chapter 2.4 Electronic Structure of the Transition Metals Chemistry Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. A metal that is located in between group 2 and group 3 (labelled as group. Transition Metals Short Form.
From www.youtube.com
Position of Transition Metals in the Periodic Table (91 GCSE Chemistry Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. typical properties of transition metals the existence of more than one oxidation state for each element in its. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general. Transition Metals Short Form.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. this page explains what a. Transition Metals Short Form.
From app.jove.com
Transition Metals Electron Configurations and Properties Concept Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. typical properties of transition metals the existence of more than one oxidation state for each element in its. A metal that is located in between group 2 and group 3 (labelled as group. Transition Metals Short Form.
From www.slideserve.com
PPT Atomic Electron Configurations and Chemical Periodicity Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. typical properties of transition metals the existence of more than one oxidation state for each element in its. transition metals are defined as those elements that have (or readily form) partially filled. Transition Metals Short Form.
From www.alevelchemistrysg.com
Summary notes of Transition Metals and their compounds Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. typical properties of transition metals the existence of more than one oxidation state for each element. Transition Metals Short Form.
From utedzz.blogspot.com
Periodic Table Labeled Transition Elements Periodic Table Timeline Transition Metals Short Form this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. this page explains what a. Transition Metals Short Form.
From www.savemyexams.com
Typical Properties of Transition Metals AQA GCSE Chemistry Revision Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. typical properties of transition metals the existence of more than one oxidation state for each element in its. A metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly. Transition Metals Short Form.
From www.youtube.com
Properties of the TRANSITION METALS Periodic Table YouTube Transition Metals Short Form transition metals are defined as those elements that have (or readily form) partially filled d orbitals. this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. A metal that is located in between group 2 and group 3 (labelled as group. Transition Metals Short Form.